Case Study
Medical Device Sealing Solutions: What Designers and Manufacturers Need To Know, Avoid And Consider When Planning Seals In A Medical Device
Medical seals are one of the most important components in many medical devices.
While small in cost, seals have a profound affect on the function of a medical device and the outcome of a medical procedure.
Engineered sealing solutions have advanced to meet the new medical device designs due both to new materials and to new processes for producing these seals. An understanding of the fundamentals of seal design, the tools available to assist in the manufacturing process and pitfalls to avoid will help in achieving a successful seal and medical device outcome.
Talk to Our Product Development Team About Your Medical Seal Challenges Now
Classifying the three basic medical seal designs
When approaching a new seal design, It is important to classify the seal based on its intended function. All seals fall into one of three distinct groups. While certain applications may combine more than one group, there is always one that is dominant. The three basic seal designs are:
- Static – seal applications where there is no movement.
- Reciprocating – seal applications where there is linear motion.
- Rotary – seal applications where there is rotation.
New advances in trocar designs incorporating specialized seals allow multiple instruments to be inserted in the single trocar.
Static seal applications are the most common and include those that prevent fluids and drugs from escaping into or out of a medical device. The seal design can range from basic O-rings to complex shapes. Static seals can be found in the broadest range of medical devices from pumps and blood separators to oxygen concentrators.
A reciprocating seal application with linear motion would include endoscopes that require trocar seals. These trocar seals are complex in design and allow the surgeon to insert and manipulate instruments to accomplish the medical procedure. These procedures range from relatively simple hernia repairs to the most difficult cardiac procedures. All of these minimally invasive surgeries employ endoscopes with seals that rely on seal stretch, durability and ability to retain shape during lengthy and arduous procedures. This particular seal application combines both reciprocating and rotary motion with the main function being linear motion.
A rotary seal application most commonly includes O-rings used to seal rotating shafts with the turning shaft passing through the inside dimension of the O-ring. Systems utilizing motors such as various types of scanning systems require rotary seals but there are many other non-motorized applications that also require rotary seals. The most important consideration in designing a rotary seal is the frictional heat buildup, with stretch, squeeze and application temperature limits also important.
Function Of A Particular Medical Seal Design
What is the function of the seal? It is important to identify specifically if the design must seal a fluid and be impermeable to a particular fluid.
Or will the seal transmit a fluid or gas, transmit energy, absorb energy and/or provide structural support of other components in device assembly.
All of these factors and combinations need to be thoroughly examined and understood to arrive at successful seal design.
A Medical Seal’s Operating Environment
In what environment will a seal operate? Water, chemicals and solvents can cause shrinkage and deformation of a seal. It is important therefore to identify the short and long term effects of all environmental factors including oxygen, ozone, sunlight and alternating effects of wet/dry situations. Equally important are the effects of constant pressure or changing pressure cycle and dynamic stress causing potential seal deformation.
There are temperature limits in which a seal will function properly. Depending on the seal material and design, a rotary shaft seal generally would be limited to an operating temperature range between -30° F and +225°F. To further generalize, the ideal operating temperature for most seals is at room temperature.
Expected Seal Life — How Long Must The Seal Perform Correctly
What is a reasonable life expectancy for a particular seal? To determine a logical answer, one must also determine factors such as stretch before breaking (high ultimate elongation). High modulus or resistance to deformation is another condition to estimate. Seal squeeze is another factor to consider for both function and life expectancy. For most rotational applications, O-ring squeeze should be kept to as little as 0.002 inches using an O-ring outer diameter at least 5% larger than the gland. The less squeeze applied minimizes potential heat build up and prolongs seal life.
Another factor to consider is resistance to set under extensive loads. Also dimensional changes over time and/or embrittlement in the presence of heat or fluids can impact performance and seal life.
Interrelated Factors Effecting Medical Seal Performance And Longevity
All three seal types are subject to multiple factors with interrelationships becoming important and often quite complex. Any combination of the factors discussed above will affect performance and can be influenced even more by such conditions as the surface finish on metal parts, use of or absence of lubrication, pressure, shock and reciprocating loads within the system and as well as system cycling speed.
Because of these complex interrelationships, it is important to seek out experienced help when designing a new medical seal application. Successful medical seal design is a constantly evolving technology with many tradeoffs and new innovations.
Medical Seal Material Selection
In approaching a new medical seal design, material selection is key to product performance. There is no substitute for experience when making compound evaluations and both custom molders and material suppliers can provide invaluable help early in the design process.
There are dozens of compounds available for a seal design not all of which are FDA compliant. All compounds are identified by three classifications. The first is by chemical term, second by an ASTM designated abbreviation and third by a polymer trade name. An example of these three descriptions is the widely used compound, silicone. Silicone is the material’s chemical name; its ASTM designated abbreviation is VMQ, PMQ and PVMQ, and its trade name among others are Plioflex® and Stereon®.
Chemical Terms, Abbreviations, Trade Names
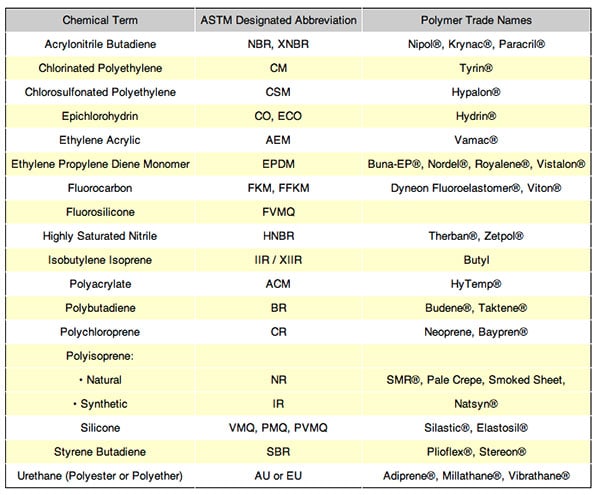
The many other available seal materials such as ethyhlene acrylic and polyubutadiene are similarly identified and classified. All compounds can be modified with the addition of other materials and/or with changes in the molding and manufacturing process to enhance a particular desired feature.
It is important to note that certain compounds are USP Class VI, ISO-10993 and FDA compliant and may be required for specific medical applications. The supplier chosen for a particular seal should be a certified supplier of that compound to qualify for a particular seal project. Typical medical seals that require ISO-10993 and FDA compliant compounds for medical applications include: medical valves, medical pumps, medical connectors, diaphragms, plunger tips, medical disposables, lab equipment, medical diagnostic products and surgical instruments.
Medical seal material selection may appear daunting, which is why we have created an online material selection tool to guide you. Explore the material selection tool for your medical seal.
Tools To Use For Correct Medical Seal Design – Finite Element Analysis
One of the most important tools for designers is the use of finite element analysis (FEA). Unlike frictional analysis where everything must be empirically tested, FEA’s can accurately predict deformation and ultimate failure of a material. Although FEA is a common tool, it is mostly used in the analysis of stiff materials like metal or plastics. Seal applications are different. Seal designs use rubber where extreme elongation, deformation and bounce back are the most important element of the part design.
This requires the use of a special type of FEA called non-linear FEA. With non-linear FEA, the seal designer creates a series of iterative seal designs that can be quickly tested. A typical FEA output appears as a video. The seal, it’s housing, and the instrument are all represented and are viewable to see what will actually happen to the seal in a working assembly. After a series of iterations are tested with FEA, it is customary to confirm the final output with prototype seals for further evaluation.
Testing The Medical Seal Design
There are many tests available for evaluating a seal design’s performance characteristics before going into production. Since surface friction is one of the most important variables affecting seal performance, testing friction is discussed here. Friction is a very complex subject affected by many variables including the lubrication state, material modulus, surface finish, temperature, geometry of the part and amount and direction of relative forces. Seal designers are therefore very focused on friction reduction.
When there is a force pressing two surfaces together and they are moving past each other as in a seal, it is impossible to calculate or predict the frictional force with accuracy. It can only be measured through experiment and the results are expressed as a coefficient of friction (COF). COF is what is used for comparison as it is a sealing system measurement rather than a measurement of the property of the material. In order to accurately measure COF, we use ASTM D1894 as a standardized test.
Dynamic Versus Static Coefficient of Friction (COF)
Dynamic and static seal differences in coefficient of friction (COF) vary greatly. The energy required to start motion is different than the energy that it takes to maintain motion. The energy required to start motion is called static COF. The energy it takes to maintain motion is called dynamic COF. The difference between static and dynamic COF will vary tremendously by material and application.
Note that the COF and relative difference between static and dynamic COF can be substantially reduced by surface textures, surface coatings and the presence of fluid. In most dry seals, for example, there is usually a stick/slip action where the seal flexes to accommodate a mating surface’s movement and then pops back to a stable state. If there is fluid present, then the cannula may “hydroplane” resulting in a significantly reduced COF.
Surface finishes and coatings of the material can substantially reduce the COF. Intuitively, we think that a rougher surface has greater friction. While this is true for large surfaces, this is not the case on a micro scale. In many applications, a matte finish can greatly reduce the amount of friction. This is because the surfaces begin to ride on top of each other. However, attention must be paid to ensure surface finish does not contribute to leakage around the seal. (Illustration Three)
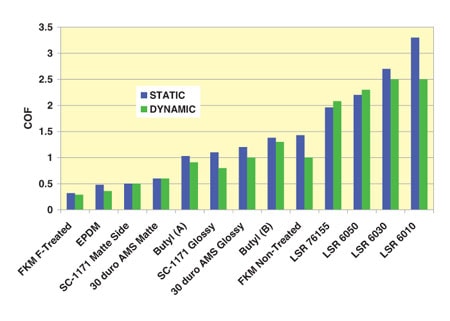
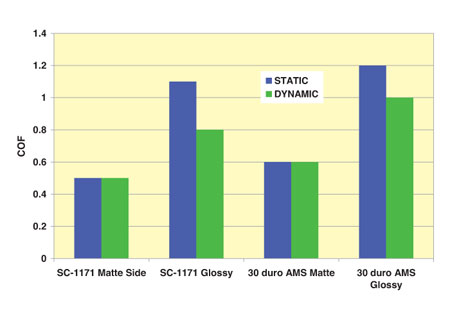
The greatest friction reduction is accomplished by surface treatment or coatings applied to the material to reduce the COF. Not only will this reduce the COF in general, it also reduces the difference between the static and dynamic COF.
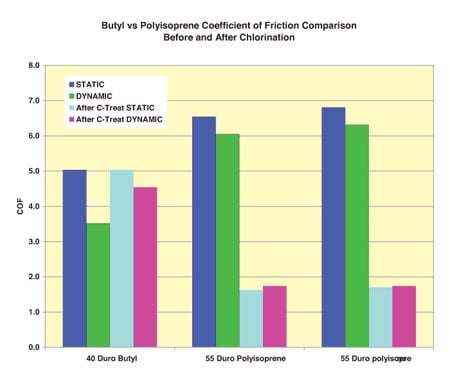
Determining what process or coating will be applied is completely dependent upon the materials selected for the seal and mating part. An example can be seen in (Illustration Four) Note that chlorination of Butyl does not result in the friction reduction seen with chlorination of the Polyisoprene. This is because the chemical structure of Butyl polymer is non-reactive with the chlorination process. Not only will the selection change depending upon the type of elastomer, it is also important to consider biocompatiability and shelf life. That being said, biocompatible forms of PTFE, Parylene™, plasma treatment, chlorination and other proprietary coating processes can reduce seal friction by up to 90%.
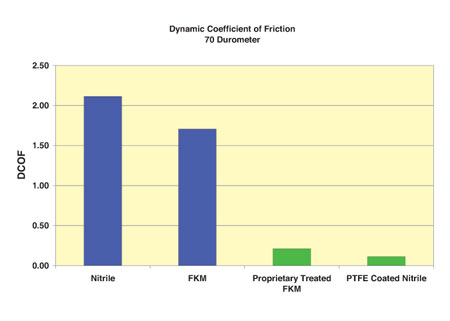
Successful Medical Seal Applications
Medical seal designs are best solved using a science-based approach. This includes analysis of material structures, their properties and how processing changes them and how the materials will perform in an application. Following the guidelines above, it requires controlling processes to ensure seal compliance and validation while producing the seals on time, on budget and in compliance.
Example real world seal applications designed and manufactured using the science-based approach include the following:
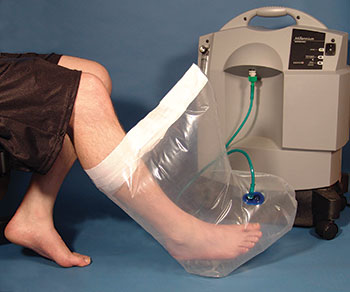
Check Valve Medical Seals Regulate Oxygen Flow In Topical Oxygen Therapy Device
This topical oxygen therapy system provides an airtight cavity of oxygen surrounding the wound so that it heals faster. A combination check valve/relief valve is controlled by a double silicone diaphragm seal in the middle of the assembly. The check valve stops the flow of oxygen out of the bag if the patient disconnects it from the oxygen source. The relief valve maintains the inflation oxygen pressure inside the bag and maintains an optimal pressure setting.

Duckbill Seals For Tocars Used In Endoscopes For Minimally Invasive Medical Surgeries
The two trocars shown have duckbill seals centered at one end. Instrument insertion is made through the seal slit that is perfectly sized and centered. The seal slit facilitates instrument insertion at the start of the surgical procedure. The specially formulated polyisoprene material is very pliable with good memory so that it stretches properly and holds shape around the inserted instrument during the surgical procedure, which may be lengthy.
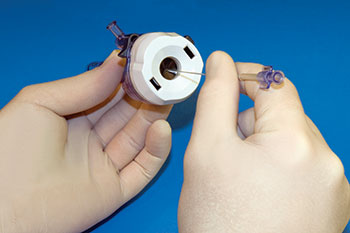

Bidirectional Medical Seals Handle Opposing Pressures In IV Systems
Used in devices for fluid delivery applications such as antibiotics, saline and pain reducers, these bidirectional seals handle opposing pressures unlike conventional seals. The seals have a double-lip configuration: the lip facing up contains downward fluid pressure while the lip facing down contains the upward, or negative fluid pressure. Specially formulated of EPDM compounds according to the fluid delivery requirements, these double-lip seals prevent leaks and facilitate accurate drug administration.


Thin-Walled Diaphragm Seals Provide Filtering And Dispensing Functions
These thin-walled diaphragms are used in a wide range of healthcare applications from dispensing and fluid filtration systems to purification systems. They are molded of a specially formulated liquid silicone rubber (LSR). They have long term seal life where temperature variation and resistance to aging are important design considerations. These seals also have good electrical insulating characteristics and resist UV radiation and weathering.
Seal-Coupler Is Key to Pneumatic Actuation Of Automated Pill Dispensing System
High speed, automated pill dispensing at pharmacies is facilitated with a unique seal coupling device. Seal couplers are connected to pill dispensing cells in the automated system that are pneumatically actuated providing the right pill prescription and an accurate pill count. The seal coupler is an eight component assembly incorporating a lip seal molded of a proprietary elastomer providing high wear resistance and leak-proof operation.
Summary
Not only are seals important in medical device applications, they are often the very heart of the device and its successful use. Original equipment manufacturers should follow a scientific, data-based approach for seal development. Selecting the material, designing the part, testing and validating the designs are all essential steps in a successful seal program. Such a program requires the early assistance of companies with deep knowledge and experience in rubber materials and sealing applications. This requires a team approach to achieve a common goal.
See How We Can Solve Your Medical Seal Challenges
Print-Ready Case Study
Download this medical seal case study in PDF format.
